Non-coding RNAs in cardiac arrhythmia
Team: Dr. Dena Esfandyari, Gheo Idrissou, Dr. Andrea Welling, Urszula Kremser, Sabine Brummer, Anton Bomhard, Hannah Britzelmeier, Alora Marks
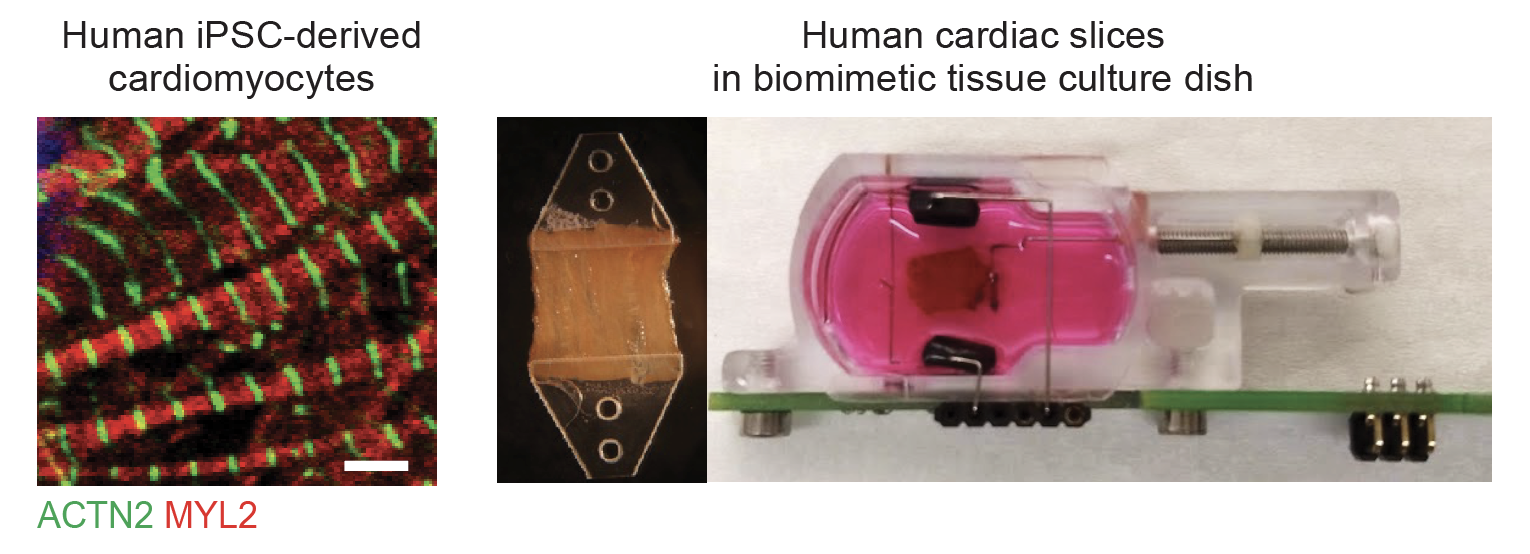
Ventricular arrhythmias contribute to 75–80% of cases of sudden cardiac death, a major cause of death in developed nations. New therapeutic interventions for such cardiac arrhythmia are urgently needed since current pharmacological treatments have met considerable challenges. Non-coding RNAs hold high promise as targets for new cardiovascular therapies, with substantial progress made in recent years (1).
Our research focuses on the identification and characterization of non-coding RNAs, e.g., microRNAs and long non-coding RNAs (lncRNAs), that are linked to cardiac rhythm-related genes (2), ultimately aiming to leverage their potential as therapeutic modulators of human cardiac arrhythmias.
Over the past few years, we developed a multidisciplinary toolbox to tackle this research question: for disease modeling, we use patient-specific induced pluripotent stem cell-derived cardiac myocytes (hiPSC-CMs), precision-cut slices from the human heart and various mouse models of cardiovascular disorders. In these models, we manipulate the level of candidate non-coding RNAs using synthetic nucleic acids, adeno-associated viruses (AAVs), and CRISPR-based approaches. Utilizing various measurement techniques, including FRET microscopy, multielectrode arrays (MEA), and electrocardiography (ECG), we determine the action potential abnormalities. We employ state-of-the-art transcriptomics, including single-cell RNA-seq, to decipher the molecular mechanisms underlying non-coding RNA control of cardiac rhythm.
Using this methodology, we have recently characterized miR-365 as a primary microRNA that regulates repolarizing ion channels and ameliorates action potential abnormalities in hiPSC-CM models of QT syndrome (2). We are currently broadening the focus of our analyses to include promising lncRNA candidates that, supported by the genetic and epigenetic evidence we have gathered, appear to play a significant role in cardiac electrophysiology.
Reference:
- Laggerbauer B, Engelhardt S. MicroRNAs as therapeutic targets in cardiovascular disease. J Clin Invest. 2022;132. doi:10.1172/JCI159179(link is external)
- Esfandyari D, Idrissou BMG, Hennis K, Avramopoulos P, Dueck A, El-Battrawy I, Grüter L, Meier MA, Näger AC, Ramanujam D, Dorn T, Meitinger T, Hagl C, Milting H, Borggrefe M, Fenske S, Biel M, Dendorfer A, Sassi Y, Moretti A, Engelhardt S. MicroRNA-365 regulates human cardiac action potential duration. Nat Commun. 2022;13:220. doi:10.1038/s41467-021-27856-7(link is external)
Related publications:

